PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions.
GENERAL DESCRIPTION & APPLICATIONS
- Cyanic acid: H-N=C=O or H-O-C��N
- Fulminic acid: (H-C=N-O) or H-C��N-O
- Isocyanic acid: H-N=C=O
- Cyanuric acid: HOC(NCOH)2N
- Biuret: (NH2)CO)2 NH
Cyanic acid hydrolyses to ammonia and carbon dioxide in water. The salts and esters of cyanic acid are cyanates. But esters of normal cyanic acid are not known. The salts and esters of isocyanic acid are isocyanates. The isocyanate group reacts with the hydroxyl functional group to form a urethane linkage. Diisocyanates (or polyisocyanates) are monomers for polyurethane production. Polyurethane is made from a variety of diisocyanates in conjunction with polyether and polyester polyols as co-reactants by addition polymerization which needs at least two -N=C=O groups. Polyurethanes are widely used in the manufacture of flexible and rigid foams, fibres, coatings, and elastomers. If isocyanate monomer is polymerized with amine group, polyurea is produced. Cyanates (or Isocyanates) are readily reacts with various form of amine (including ammonia, primary-, secondary-amines, amides and ureas) and hydroxyl functional group. They are used in the synthesis for the target molecules such as pharmaceuticals, pesticides, textile softener, lubricants and industrial disinfectants. They can convert to polycyclic compounds such as hydantoins and imidazolons. They are used as plastic additives and as heat treatment salt formulations for metals.
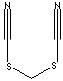
Thiocyanate is a salt or ester of thiocyanic acid (HSCN). Aqueous solutions of thiocyanic acid , also called sulfocyanic acid, are very strong acids of the equilibrium mixture of thiocyanic and isothiocyanic. Thiocyanates are bonded through the sulfur(s) with the structure R-S-C��N or the isomeric R-N=C=S (isothiocyanates). Thiocyanates are bonded through the sulfur(s) which replace for the oxygen (O) atom. Thiocyanates are the sulfur analog of isocyanates. Organic as well as metallic thiocyanates [CuSCN, Ca(SCN)2, NaSCN, KSCN] are very versatile compounds. Isothiocyanates act as electrophiles with the carbon atom as the electrophilic center. They have wide range of applications as an intermediate to create a desired chemical compound through a series of chemical reactions for the industrial field includingf fungicide, bactericide, wood preservative, pharmaceutical and plastic and rubber. Some isothiocyanates are used in freezing solutions, fabric dyeing, electroplating, steel pickling, printing, and corrosion inhibitor against acid gases. They are used in photography industry as a stabilizer or accelerator. Certain isothiocyanates, such as phenethyl isothiocyanate and sulforaphane are known as chemopreventive agents against the development and proliferation of cancers. Allyl isothiocyanate, called mustard oil, shows also anti-tumor and anti-oxidant properties by inducing the activity of phase II detoxification enzymes in the urinary bladder.
Methylene Bis(thiocyanate) is used in formulating biocides. It is used as a preservatives for coatings, slurries and to control microbial fouling in paper mills , oil field and leather process. It is used in water treatment process.
APPEARANCE
ASSAY
98.0% min